原文链接:

文章链接:LIFE-A-0002原文
作者:
Ali Ellebedy是一名华盛顿大学医学院的免疫学家[2],研究方向主要包括:①确定调节记忆 B 细胞异质性和命运的细胞和分子机制、②了解针对快速演变的病原体(如流感病毒)激发广泛中和 B 细胞反应的规则。Ellebedy团队的代表性成就包括在轻症新冠肺炎康复者的骨髓中发现了新冠病毒特异性的长寿骨髓浆细胞(BMPCs,一种效应B细胞)[3]等新冠疫苗的相关研究[4]。
摘要原文及简述:
PartI:Infuenza viruses remain a major public health threat. Seasonal infuenza vaccination in humans primarily stimulates pre-existing memory B cells, which diferentiate into a transient wave of circulating antibody-secreting plasmablasts. This recall response contributes to ‘original antigenic sin’—the selective increase of antibody species elicited by previous exposures to infuenza virus antigens. PartII:It remains unclear whether such vaccination can also induce germinal centre reactions in the draining lymph nodes, where diversifcation and maturation of recruited B cells can occur. PartIII-1:Here we used ultrasound-guided fne needle aspiration to serially sample the draining lymph nodes and investigate the dynamics and specifcity of germinal centre B cell responses after infuenza vaccination in humans. PartIII-2:Germinal centre B cells that bind to infuenza vaccine could be detected as early as one week after vaccination. In three out of eight participants, we detected vaccine-binding germinal centre B cells up to nine weeks after vaccination. PartIII-3:Between 12% and 88% of the responding germinal centre B cell clones overlapped with B cells detected among early circulating plasmablasts. These shared B cell clones had high frequencies of somatic hypermutation and encoded broadly cross-reactive monoclonal antibodies. PartIII-4:By contrast, vaccine-induced B cell clones detected only in the germinal centre compartment exhibited signifcantly lower frequencies of somatic hypermutation and predominantly encoded strain-specifc monoclonal antibodies, which suggests a naive B cell origin.Some of these strain-specifc monoclonal antibodies recognized epitopes that were not targeted by the early plasmablast response. PartIII-5:Thus, infuenza virus vaccination in humans can elicit a germinal centre reaction that recruits B cell clones that can target new epitopes, thereby broadening the spectrum of vaccine-induced protective antibodies.
- 1.背景介绍:流感病毒Influenza是重大威胁。流感疫苗的原理是刺激人体内已存在的记忆B细胞(memory B)是其分化为循环浆母细胞(plasmablasts)[5],这种现象有助于”抗原原罪现象“[6](机体因为曾经感染过旧抗原而对微小变化的新抗原反应下降)
- 2.提出问题:疫苗的作用是否也能诱导引流淋巴结(draining lymph nodes)中生发中心(germinal centre)反应?
- 3.实验及结论:
- (1)实验:在这里,作者使用超声波引导的fne针抽吸对引流淋巴结进行连续采样,并研究人类接种流感疫苗后生发中心B细胞反应的动力学和特异性。
- (2)结论1:最早可以在接种疫苗一周后检测到与流感疫苗结合的生殖中心B细胞。在八分之三的参与者中,我们在接种疫苗后九周内检测到了与疫苗结合的生发中心B细胞。
- (3)结论2:12%至88%的有反应的生发中心B细胞克隆与在早期循环浆母细胞(circulating plasmablasts)中检测到的B细胞重叠。这些共享的B细胞克隆具有高频率的体细胞超突变,并编码广泛的交叉反应单克隆抗体。
- (4)结论3:相比之下,仅在生发中心室检测到的疫苗诱导的B细胞克隆表现出明显较低的体细胞超突变频率,并且主要编码菌株特异性单克隆抗体,这表明B细胞起源于幼稚的B细胞。这些菌株特异性单克隆抗体中的一些识别早期浆母细胞反应未靶向的表位。
- (5)结论4:因此,在人类中接种流感病毒疫苗可以引发生发中心反应,招募可以靶向新表位的B细胞克隆,从而拓宽疫苗诱导的保护性抗体的范围。
- 4.问题回答:在人类中接种流感病毒疫苗可以引发生发中心反应,招募可以靶向新表位的B细胞克隆,从而拓宽疫苗诱导的保护性抗体的范围。(也就代表着疫苗会对生发中心产生影响,和免疫原罪好像没关系)
正文简述:
实验细节一:FNA采样
- Q1:作者使用的样本有哪些?
A:作者使用了8名志愿者在接种四价流感疫苗(QIVs)5个时间节点的外周血(blood)和同侧腋窝淋巴结(FNA)数据;并对所有时间点的全样本进行了单细胞 RNA 测序(scRNA-seq)分析,以全面检测其细胞组成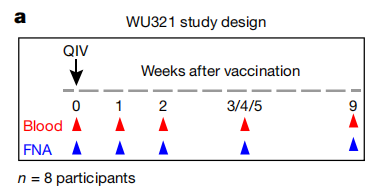
- Q2:作者怎样提取出挑选其中的细胞?
A:我们对接种疫苗一周后在 PBMCs 中的单个 PBs 和FNA中的GC(只有FNA中会存在GC)进行了分拣,并将相应的免疫球蛋白基因表达为重组单克隆抗体
We sorted single PBs isolated from PBMCs one week after vaccinationand expressed the corresponding immunoglobulin genes as recombinant monoclonal antibodies(Extended Data Figs. 1d, 3a). We also generated monoclonalantibodies from single GC B cells sorted irrespective of their binding to HA probes from three time points for each of the three participants.
实验细节二:序列操作
- Q1:作者如何进行聚类?
A:利用单克隆抗体的 Vh 基因信息和bulk-sorted得到的PB信息来综合聚类得到clonal。具体的:- 聚类原理:clonal lineages were inferred based on productively rearranged heavy chain sequences using hierarchical clustering with single linkage
- 进化原理:Clonal overlap between week 1 PBs from blood and the GC B cells from later weeks was determined by the presence of sequences from both compartments in the same B cell clone
- SHM计算:calcObservedMutations function from SHazaM v.0.1.11
- 建树原理:Phylogenetic trees for responding B cell clones were constructed using IgPhyML v1.1.0 (ref. 40) with the HLP19 model
- 结论1:发现QIV-binding的GC更易溯源,且GC和PB重叠的B细胞有着更高的SHM比例和广谱性
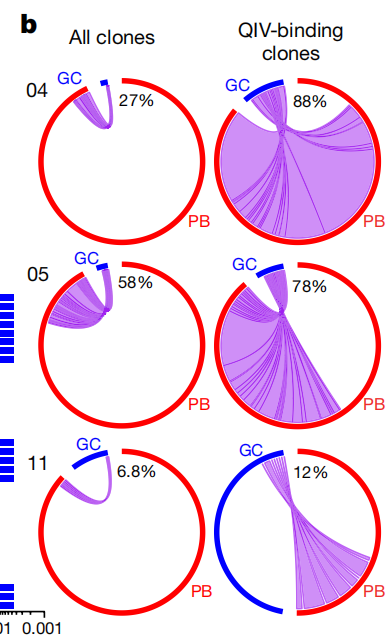
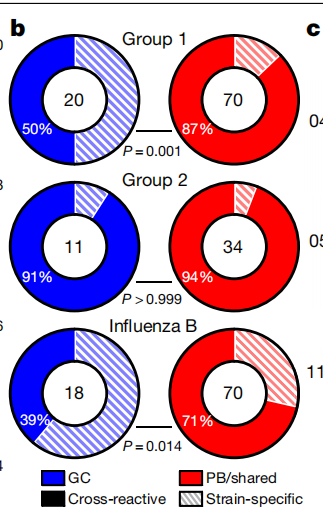
- 结论2:除此之外还发现了一些找不到PB溯源的GC,它们的SHM以及广谱性都较差,倾向于特异性(Strain-specific),可能是Naive B起源
- 广谱的定义:Antibodies are considered broadly cross-reactive if they recognize HAs from H1N1 or H3N2 strains that no longer circulate in humans
- 浆母细胞与生发中心细胞:被TFH激活的B细胞部分迁移到髓质索(medullary cord),5天左右以后扩增形成原发灶(primary focus)。这些细胞一般都是具有较高亲和力的IgM分泌原浆细胞(plasmablast),它们是病原体感染第一阶段的体液免疫细胞。通常这些细胞寿命较短,不能形成成熟浆细胞(plasma cells)。另外大多数被TFH激活但亲和力不够高的B细胞会进入滤泡,在这里大量增殖形成生发中心。含有GC的滤泡被称为次级滤泡(secondary lymphoid follicle)。[8]
参考链接:
- [1].原文:Human germinal centres engage memory and naive B cells after influenza vaccination | Nature
- [2].阿里教授主页:Ali Ellebedy, PhD | Pathology & Immunology | Washington University in St. Louis (wustl.edu)
- [3].BMPC综述:《自然》:新冠研究有重大进展!科学家首次发现新冠康复患者体内存在长寿骨髓浆细胞,对抗新冠病毒的长效体液免疫确实存在丨科学大发现_抗体 (sohu.com)
- [4]Ellebedy对新冠疫苗的研究:Nature Cell三连发:Ali H. Ellebedy团队揭示新冠mRNA疫苗接种后的长效保护_公司新闻_丁香通 (biomart.cn)
- [5]循环B细胞:免疫细胞注释-3:B细胞 – 简书 (jianshu.com)
- [6]抗原原罪:科普下疫苗中可能出现的抗原原罪和ADE反应 – 知乎 (zhihu.com)
- [7]FNA定义:【医技科普】甲状腺细针穿刺抽吸活检(FNA),您了解吗? (sohu.com)
- [8]浆母细胞与生发中心的关系:体液免疫(三):B细胞的前世今生(下) – 知乎 (zhihu.com)
